FDA Approves Foam to Treat Scalp Eczema in Children and Adults
[ad_1]
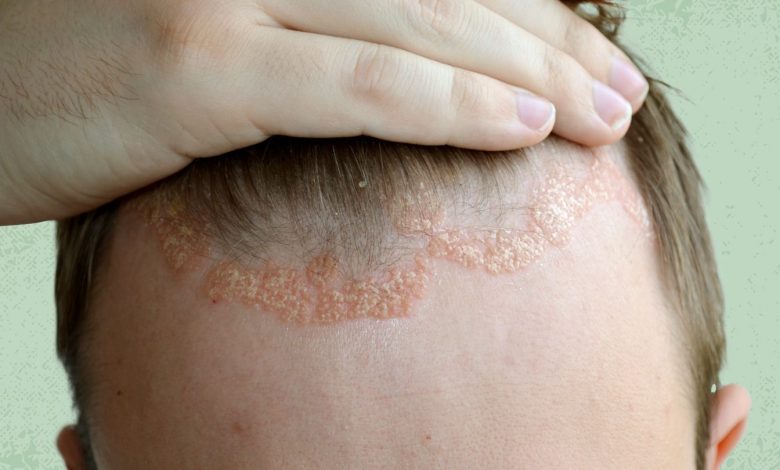
The U.S. Food and Drug Administration (FDA) has approved the topical foam Zoryve (roflumilast) to treat an inflammatory skin condition known as seborrheic dermatitis, commonly called scalp eczema, that can cause a scaly, itchy rash on the scalp and skin.
Zoryve foam is cleared to treat seborrheic dermatitis in adults and children 9 years and older and will be available in the United States by the end of January, its developer, Arcutis Biotherapeutics, said in a statement.
“There has been a real struggle with disease clearance and treatment adherence due to lack of efficacy, difficulty treating certain body areas, inconvenient treatment regimens, and concerns about safety with long-term use,” said Patrick Burnett, MD, PhD, the chief medical officer at Arcutis, in the statement. “Zoryve foam is a once-daily, steroid-free topical treatment that can be used anywhere on the body, including hair-bearing areas, with no limitation on duration of use.”
Seborrheic dermatitis is a chronic type of eczema that typically appears on the scalp and other areas of the skin where there are lots of oil-producing sebaceous glands. It causes an itchy rash on the head known as cradle cap in babies. In adults, milder cases of seborrheic dermatitis can cause dandruff. More severe cases can lead to thickened skin patches, and persistent scratching can also cause serious skin infections.
Black people are more prone to this condition, and it’s more common in men than in women, according to the American Academy of Dermatology (AAD). On darker skin tones, the rash can look pink or purple and appear lighter than surrounding skin, while it looks reddish and darker than nearby tissue on individuals with paler skin tones, according to AAD.
Common Symptoms of Scalp Eczema
Common symptoms of seborrheic dermatitis include dry, flaky skin and rashes accompanied by itchy, burning sensations, according to AAD. The condition appears most often on the scalp, but can also be found on the face, eyelids, area around the ears, and in places on the body where skin folds develop or skin rubs together, like the armpits, belly button, or area underneath the breasts.
While treatment regimens can vary based on where on the body seborrheic dermatitis develops, milder cases that often start on the scalp are initially treated with dandruff shampoo, according to AAD. More severe cases are treated with topical corticosteroids, which AAD recommends using for as little time as possible because of the potential for skin damage and the risk of side effects like high blood pressure, cataracts, and glaucoma with long-term use.
“Chronic inflammatory conditions have historically been challenging to treat because topical anti-inflammatory steroid creams cannot be used chronically safely,” says Chris Adigun, MD, a dermatologist at the Dermatology and Laser Center of Chapel Hill in North Carolina.
“Zoryve is therefore a better option for this chronic condition because it can be used regularly without the risks of chronic topical steroid use, which is most notable for causing thinning of the skin,” Dr. Adigun says.
How Zoryve Works
Zoryve reduces inflammation that contributes to the development of seborrheic dermatitis. The drug was previously approved to treat plaque psoriasis, which, like seborrheic dermatitis, develops when inflammation causes changes to the skin.
In a late-stage clinical trial used to seek FDA approval, researchers randomly assigned 304 people with seborrheic dermatitis to use Zoryve for eight weeks, while another 153 people with the condition were given a placebo foam.
Roughly 4 in 5 patients who received the drug experienced significant improvement in their skin by the end of the study, and skin cleared up completely for half of them. In addition, more than 60 percent of people in the Zoryve group also experienced significant reductions in itching, sometimes within just a day or two of starting to use the medicine, according to results reported at the 2023 American Academy of Dermatology meeting.
There were very few treatment-related side effects with Zoryve, according to data reported by the meeting. The most common side effects were urinary tract infections, nausea, pain at the application site, and sinusitis.
“Overall, Zoryve presents several advantages for the treatment of seborrheic dermatitis, including its proven efficacy, user-friendly application, favorable safety profile, and anticipated accessibility,” says Danilo C. Del Campo, MD, a dermatologist at the Chicago Skin Clinic. “Seborrheic dermatitis, a prevalent and often stubborn dermatological condition that affects a considerable portion of the population, can now be treated more effectively with the approval of Zoryve.”
[ad_2]




